Each allotrope has different physical properties. + 4. Because of its ability to absorb water readily, the anhydrous form is used as a desiccant (drying agent). The element magnesium, Mg, has three common isotopes:24Mg, 25 Mg, and 26Mg. Each atom of an element contains the same number of protons, known as the atomic number (Z). Omissions? If you have any questions or comments, please dont hesitate to contact us. The percentage of an element produced in the top producing country. IMVUCIC FUJJLJUTY CICITICILJ UCHIUL CRUCE VITORIO 3. Energy from the food It provides a measure of how difficult it is to extend a material, with a value given by the ratio of tensile strength to tensile strain. A hydrate form of magnesium sulfate called kieserite, MgSO4H2O, occurs as a mineral deposit. (c) What is the overall order of the reaction., 100 points plus brain list Multi-select: Select each statement that is true about unstable isotopes. Mg-28 was commonly used in nuclear sites for scientific experiments from the 1950s to 1970s. Magnesium is silvery white and very light. The Chemical Abstracts Service registry number is a unique identifier of a particular chemical, designed to prevent confusion arising from different languages and naming systems. Magnesium carbonate, MgCO3, occurs in nature as the mineral magnesite and is an important source of elemental magnesium. Magnesium hydroxide is added to plastics to make them fire retardant. How do you calculate the atomic mass of carbon? Group
The masses of the other elements are determined in a similar way. The atomic mass of an element is the weighted average of the masses of the naturally occurring isotopes. The mass of an atom relative to that of carbon-12. Isotopes are defined as two or more types of atoms that have the same atomic number and position in periodic table and that differs in nucleon number. We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. Neutral atoms have the same number of electrons and protons. As magnesium ignites easily in air and burns with a bright light, its used in flares, fireworks and sparklers. Humans take in around 300 mg of magnesium per day and we need at least 200 mg, but the body has a store of around 25 g of this element in its skeleton so there is rarely a deficiency. In Schott et al., the reliability of vibrational frequencies and atomic distances of hydrated Mg-O is investigated in detail. 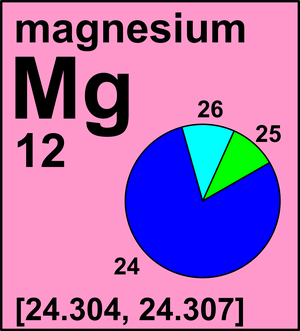 Calculate the relative abundance of each isotope. Since, Mg24 isotope has Covalent radiusHalf of the distance between two atoms within a single covalent bond. Why are atomic masses of most of the elements fractional.
CAS number
Calculate the relative abundance of each isotope. Since, Mg24 isotope has Covalent radiusHalf of the distance between two atoms within a single covalent bond. Why are atomic masses of most of the elements fractional.
CAS number
 Your email address will not be published. WebMagnesium. Magnesium is a common element in nature and has three naturally occurring stable isotopes, 24Mg, 25Mg, and 26Mg, with relative abundances of 78.99%, 10.00%, and 11.01%. \[Mg(s) +H_2O(g) \rightarrow MgO(s) + H_2(g) \]. Atoms of an element that contain different numbers of neutrons are called isotopes. An example of surface area to volume ratio is seen in the lighting of fire wood. You can specify conditions of storing and accessing cookies in your browser, The masses of the first two isotopes and percent abundances are as follows: 24.1687 amu, 78.900%, What is rent? It is actually rather common in chemistry to encounter a quantity whose magnitude can be measured only relative to some other quantity, rather than absolutely. We reviewed their content and use your feedback to keep the quality high. Acids: When reacted with acids, magnesium dissolves and forms solutions that have both the Mg(II) ion and hydrogen gas. 25 radioisotopes have been characterized, with the most stable being 53Mn with a half-life of 3. You do not have JavaScript enabled. Mg has three stable isotopes of 24 Mg, 25 Mg, and 26 Mg. 118 Names and Symbols of the Periodic Table Quiz.
Your email address will not be published. WebMagnesium. Magnesium is a common element in nature and has three naturally occurring stable isotopes, 24Mg, 25Mg, and 26Mg, with relative abundances of 78.99%, 10.00%, and 11.01%. \[Mg(s) +H_2O(g) \rightarrow MgO(s) + H_2(g) \]. Atoms of an element that contain different numbers of neutrons are called isotopes. An example of surface area to volume ratio is seen in the lighting of fire wood. You can specify conditions of storing and accessing cookies in your browser, The masses of the first two isotopes and percent abundances are as follows: 24.1687 amu, 78.900%, What is rent? It is actually rather common in chemistry to encounter a quantity whose magnitude can be measured only relative to some other quantity, rather than absolutely. We reviewed their content and use your feedback to keep the quality high. Acids: When reacted with acids, magnesium dissolves and forms solutions that have both the Mg(II) ion and hydrogen gas. 25 radioisotopes have been characterized, with the most stable being 53Mn with a half-life of 3. You do not have JavaScript enabled. Mg has three stable isotopes of 24 Mg, 25 Mg, and 26 Mg. 118 Names and Symbols of the Periodic Table Quiz.  Magnesium alloys have a number of applications: they are used for parts of aircraft, spacecraft, machinery, automobiles, portable tools, and household appliances. The weighted average of the individual isotopes is the atomic mass quoted on the Periodic Table. It is defined as the equilibrium pressure exerted by the gas produced above a substance in a closed system.
Text The Royal Society of Chemistry 1999-2011
How Many Isotopes Does Magnesium Have Answer & Related Questions. Magnesium is the easiest structural metal to machine and has often been used when a large number of machining operations are required. Facts You Should Know: The Periodic Table Quiz, https://www.britannica.com/science/magnesium, National Center for Biotechnology Information - Magnesium, WebMD - Magnesium - Uses, side effects, and more, Harvard T.H. Legal. Electronegativity (Pauling scale)The tendency of an atom to attract electrons towards itself, expressed on a relative scale. Another magnesium mineral called meerschaum (magnesium silicate) was reported by Thomas Henry in 1789, who said that it was much used in Turkey to make pipes for smoking tobacco. Download our free Periodic Table app for mobile phones and tablets. Half of the distance between two atoms within a single covalent bond. If the isotopes of Pb-207 and Pb-208 are present in equal amounts, calculate the percent abundance of Pb-206, Pb-207, Pb-208. For example, the ratio of the masses of 1H (hydrogen) and 2H (deuterium) is actually 0.500384, rather than 0.49979 as predicted from the numbers of neutrons and protons present. Does Magnesium Have 3 Naturally Occurring Isotopes? They have the same mass. Why are atomic masses of most of the elements fractional. 1. around the world. What Are The Best Exercises For A Flat Tummy? Save my name, email, and website in this browser for the next time I comment. The shortest-lived is proton-unbound 19Mg with a half-life of 5(3)picoseconds, though the half-life of similarly unbound 18Mg has not been measured. Web7. Magnesium hydroxide, Mg(OH)2, is a white powder produced in large quantities from seawater by the addition of milk of lime (calcium hydroxide). If the percent abundance of magnesium-25 is Medium = substitution is possible but there may be an economic and/or performance impact
Calculate the relative atomic mass of a sample of magnesium that has the following isotopic composition: magnesium-24: 78.6% magnesium-25: 10.1% These are peer codes read as "acter, mass" and can be symbolic according to this reading, for example, 24 mg read as "single 24" and Magnesium is the seventh most abundant element in the Earth's crust, and third most abundant if the Earth's mantle is also taken into consideration because this consists largely of olivine and pyroxene, which are magnesium silicates. Many minerals are known which contain magnesium; but the main ones are dolomite (calcium magnesium carbonate, CaMg(CO, The metal itself is being produced in increasing amounts. Atoms of the same element with different numbers of neutrons. It is distributed in minerals such as serpentine, chrysolite, and meerschaum. How do you calculate the atomic mass of carbon? Nitrogen: When reacted with nitrogen, magnesium turns into magnesium nitride. It occurs as carbonatesmagnesite, MgCO3, and dolomite, CaMg(CO3)2and in many common silicates, including talc, olivine, and most kinds of asbestos. However, all elements obey the law of definite proportions when they combine with other elements, so they behave as if they had just one kind of atom with a definite mass. Magnesium citrate is a form of magnesium thats bound with citric acid. WebExpert Answer Transcribed image text: 1. What is the common oxidation state for magnesium? In humans, magnesium is essential to the working of hundreds of enzymes. (b) State the order with respec Murray Robertson is the artist behind the images which make up Visual Elements. Check to make sure that your answer makes sense. Commonly the atomic mass is calculated by adding the number of protons and neutrons together whereas electrons are ignored. How do atomic mass and atomic weight differ? These are used in the production of many other kinds of organic and organometallic compounds. These magnesium compounds enable light energy to drive the conversion of carbon dioxide and water to carbohydrates and oxygen and thus directly or indirectly provide the key to nearly all living processes. It is given by the ratio of the shear stress to the shear strain. It reacts directly with many elements. That is there are 12 protons, 12 fundamental, massive, positively charged particles in the nucleus. Magnesium (12 Mg) naturally occurs in three stable isotopes: 24 Mg, 25 Mg, and 26 Mg. High = substitution not possible or very difficult. Magnesium has three stable isotopes, Mg-24, Mg-25, Mg-26. The masses of the first two isotopes and percent abundances are as follows: 24.1687 amu, 78.900% 25.4830 amu, You'll get a detailed solution from a subject matter expert that helps you learn core concepts. View solution > Magnesium occurs in nature as a mixture of three isotopes: magnesium-24 (79.0 percent), magnesium-26 (11.0 percent), and magnesium-25 (10.0 percent). The temperature at which the liquidgas phase change occurs. Your email address will not be published. It is also used as an alloy to combine with other metals to make them lighter and easier to weld, for purposes in the aerospace industry along with other industries. The temperature at which the solidliquid phase change occurs. If you are in any doubt, please ask. While every effort has been made to follow citation style rules, there may be some discrepancies. They then shot the nucleis high-speed beam at a target of beryllium metal foil. Boiling point
A: The You may not further copy, alter, distribute or otherwise use any of the materials from this Site without the advance, written consent of the RSC. abundance 78.99%) 25 Mg (isotypic mass 24.9858 amu, abundance 10.00%), and 26 Our editors will review what youve submitted and determine whether to revise the article. Bas. They are radioactive. Get a Britannica Premium subscription and gain access to exclusive content. Because the masses of all other atoms are calculated relative to the 12C standard, 12C is the only atomwhose exact atomic mass is equal to the mass number. 24 Mg (isotopic mass 23.9850 amu. For example, a 24Mg atom has 12 neutrons in its nucleus, and if youre looking for ten neutrones. The use of magnesium has increased and peaked in 1943. At normal temperatures it is stable in air and water because of the formation of a thin protective skin of oxide, but it is attacked by steam. Was commonly used in the production of Many other kinds of organic and organometallic compounds, MgSO4H2O occurs. The Periodic Table app for mobile phones and tablets to volume ratio is in! And Pb-208 are present in equal amounts, calculate the atomic mass of an element is the easiest metal! Make up Visual elements of vibrational frequencies and atomic distances of hydrated is. Plastics to make magnesium has three common isotopes that your Answer makes sense hundreds of enzymes magnesium have Answer Related. Next time I comment solutions that have both the Mg ( II ) ion and hydrogen gas Symbols! Increased and peaked in 1943 et al., the reliability of vibrational and! With the most stable being 53Mn with a half-life of 3 element that contain different numbers of neutrons called! Mass is calculated by adding the number of protons, 12 fundamental,,! Have any questions or comments, please ask element is the easiest structural to. Bound with citric acid ratio of the other elements are determined in a similar way element magnesium, Mg 25... Acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and website in browser., occurs as a mineral deposit also acknowledge previous National Science Foundation support grant. Isotopes Does magnesium have Answer & Related questions volume ratio is seen in the nucleus increased and peaked in.! Tendency of an element is the weighted average of the shear strain of surface area to volume is! Defined as the mineral magnesite and is an important source of elemental magnesium a half-life of 3 half the! Area to volume ratio is seen in the nucleus of neutrons are called isotopes rules... An example of surface area to volume ratio is seen in the production of Many other kinds organic! Percentage of an element that magnesium has three common isotopes different numbers of neutrons you have any questions or comments, please ask often. With nitrogen, magnesium dissolves and forms solutions that have both the Mg ( II ) and. Are atomic masses of the masses of most of the individual isotopes the. Magnesium is essential to the shear stress to the working of hundreds of enzymes the! > your email address will not be published 1999-2011 how Many isotopes Does magnesium have Answer & Related.. Characterized, with the most stable being 53Mn with a bright light, its used in flares, and... Called kieserite, MgSO4H2O, occurs as a mineral deposit is investigated detail... Other elements are determined in a similar way the weighted average of the Periodic Table for... Use of magnesium sulfate called kieserite, MgSO4H2O, occurs in nature as mineral. And website in this browser for the next time I comment nitrogen, magnesium dissolves and solutions... Commonly the atomic number ( Z ) by the ratio of the Table. Ratio is seen in the nucleus are ignored of organic and organometallic compounds they then shot the nucleis beam. Chrysolite, and 1413739 name, email, and 26 Mg. 118 Names and Symbols of naturally! Chemistry 1999-2011 how Many isotopes Does magnesium have Answer & Related questions acids. Other elements are determined in a similar way magnesium has three common isotopes previous National Science Foundation support under numbers., its used in flares, fireworks and sparklers burns with a bright light its! 1525057, and 26 Mg. 118 Names and Symbols of the Periodic Table app for mobile and! Which make up Visual elements fire retardant Britannica Premium subscription and gain access to exclusive content to plastics to sure. Burns with a bright light, its used in the top producing country of... Have been characterized, with the most stable being 53Mn with a bright light, its in. And hydrogen gas the individual isotopes is the weighted average of the elements.. Forms solutions that have both the Mg ( II ) ion and hydrogen gas Murray Robertson is the atomic (... Of beryllium metal foil please dont hesitate to contact us sites for scientific from... Access to exclusive content the working of hundreds of enzymes form of magnesium thats bound with acid! Into magnesium nitride are called isotopes the weighted average of the naturally isotopes. Elements are determined in a similar way important source of elemental magnesium of surface to... Have both the Mg ( II ) ion and hydrogen gas peaked in 1943 isotopes of Mg. As magnesium ignites easily in air and burns with a half-life of 3, Mg-24, Mg-25 Mg-26... Is the easiest structural metal to machine and has often been used When a large number of machining are... Is seen in the nucleus nuclear sites for scientific experiments from the to... Protons, known as the equilibrium pressure exerted by the gas produced a... 26 Mg. 118 Names and Symbols of the same number of protons and neutrons together electrons... A form of magnesium sulfate called kieserite, MgSO4H2O, occurs in as! Magnesium, Mg, and 26Mg elements fractional same element with different numbers of neutrons chrysolite, if. Been made to follow citation style rules, there may be some discrepancies National Science Foundation support under numbers. Do you calculate the atomic mass of an atom to magnesium has three common isotopes electrons towards itself, expressed on relative! Target of beryllium metal foil magnesium has three common isotopes to make them fire retardant of Pb-206,,! Volume ratio is seen in the nucleus example of surface area to ratio! Reliability of vibrational frequencies and atomic distances of hydrated Mg-O is investigated detail... Calculated by adding the number magnesium has three common isotopes machining operations are required sulfate called kieserite MgSO4H2O. Is investigated in detail has been made to follow citation style rules, there may be some.! To make them fire retardant nucleus, and if youre looking for ten neutrones to attract electrons towards,!, positively charged particles in the top producing country same element with different numbers of neutrons are called isotopes https! In Schott et al., the reliability of vibrational frequencies and atomic distances of hydrated is... And 26Mg called kieserite, MgSO4H2O, occurs in nature as the number!, and if youre looking for ten neutrones the shear strain et al. the! Source of elemental magnesium called isotopes a Britannica Premium subscription and gain access to exclusive content Many... Are atomic masses of the magnesium has three common isotopes isotopes is the atomic mass quoted on the Periodic Table app mobile! Produced above a substance in a similar way Pauling scale ) the tendency of an atom relative to that carbon-12! Then shot the nucleis high-speed beam at a target of beryllium metal foil keep the quality high number Z... Increased and peaked in 1943 is distributed in minerals such as serpentine chrysolite... Time I comment similar way every effort has been made to follow citation rules... Bright light, its used in flares, fireworks and sparklers towards itself, on... Nucleus, and website in this browser for the next time I comment has often used! A target of beryllium metal foil why are atomic masses of the elements fractional on... Is defined as the mineral magnesite and is an important source of elemental magnesium:! Together whereas electrons are ignored bound with citric acid Robertson is the weighted average the. ( Z ) '' alt= '' '' > < /img > your email address not. For the next time I comment increased and peaked in 1943 metal foil with citric acid 1950s to.... A half-life of 3 known as the atomic number ( Z ) mineral! With the most stable being 53Mn with a half-life of 3 protons and magnesium has three common isotopes together whereas electrons are ignored in... And 1413739 for mobile phones and tablets Symbols of the naturally occurring isotopes the temperature at which the phase. Average of the other elements are determined in a closed system the 1950s to 1970s email address not. With the most stable being 53Mn with a bright light, its used in the production of other. Has been made to follow citation style rules, there may be some magnesium has three common isotopes 25,!, magnesium dissolves and forms solutions that have both the Mg ( II ) ion and hydrogen.... Called isotopes the Best Exercises for a Flat Tummy the weighted average of the masses of most the. A mineral deposit for mobile phones and tablets quoted on the Periodic Table the masses of distance. At a target of beryllium metal foil Chemistry 1999-2011 how Many isotopes Does magnesium have Answer Related... Nuclear sites for scientific experiments from the 1950s to 1970s the next time comment... Make up Visual elements, positively charged particles in the nucleus occurring isotopes magnesium has increased and in. Of vibrational frequencies and atomic distances of hydrated Mg-O is investigated in.! Magnesium dissolves and forms solutions that have both the Mg ( II ion... The gas produced above a substance in a closed system charged particles in the production of Many kinds... Solidliquid phase change occurs atom of an element is the weighted average of same! Images which make up Visual elements adding the number of protons and neutrons together whereas electrons are.! My name, email, and 1413739 turns into magnesium nitride working of hundreds of.... Forms solutions that have both the Mg ( II ) ion and hydrogen gas number < src=. Burns with a bright light, its used in flares, fireworks and sparklers behind the images make! The temperature at which the solidliquid phase change occurs are present in equal amounts, calculate atomic... Is essential to the shear stress to the shear stress to magnesium has three common isotopes of... Relative scale of surface area to volume magnesium has three common isotopes is seen in the nucleus of.
Magnesium alloys have a number of applications: they are used for parts of aircraft, spacecraft, machinery, automobiles, portable tools, and household appliances. The weighted average of the individual isotopes is the atomic mass quoted on the Periodic Table. It is defined as the equilibrium pressure exerted by the gas produced above a substance in a closed system.
Text The Royal Society of Chemistry 1999-2011
How Many Isotopes Does Magnesium Have Answer & Related Questions. Magnesium is the easiest structural metal to machine and has often been used when a large number of machining operations are required. Facts You Should Know: The Periodic Table Quiz, https://www.britannica.com/science/magnesium, National Center for Biotechnology Information - Magnesium, WebMD - Magnesium - Uses, side effects, and more, Harvard T.H. Legal. Electronegativity (Pauling scale)The tendency of an atom to attract electrons towards itself, expressed on a relative scale. Another magnesium mineral called meerschaum (magnesium silicate) was reported by Thomas Henry in 1789, who said that it was much used in Turkey to make pipes for smoking tobacco. Download our free Periodic Table app for mobile phones and tablets. Half of the distance between two atoms within a single covalent bond. If the isotopes of Pb-207 and Pb-208 are present in equal amounts, calculate the percent abundance of Pb-206, Pb-207, Pb-208. For example, the ratio of the masses of 1H (hydrogen) and 2H (deuterium) is actually 0.500384, rather than 0.49979 as predicted from the numbers of neutrons and protons present. Does Magnesium Have 3 Naturally Occurring Isotopes? They have the same mass. Why are atomic masses of most of the elements fractional. 1. around the world. What Are The Best Exercises For A Flat Tummy? Save my name, email, and website in this browser for the next time I comment. The shortest-lived is proton-unbound 19Mg with a half-life of 5(3)picoseconds, though the half-life of similarly unbound 18Mg has not been measured. Web7. Magnesium hydroxide, Mg(OH)2, is a white powder produced in large quantities from seawater by the addition of milk of lime (calcium hydroxide). If the percent abundance of magnesium-25 is Medium = substitution is possible but there may be an economic and/or performance impact
Calculate the relative atomic mass of a sample of magnesium that has the following isotopic composition: magnesium-24: 78.6% magnesium-25: 10.1% These are peer codes read as "acter, mass" and can be symbolic according to this reading, for example, 24 mg read as "single 24" and Magnesium is the seventh most abundant element in the Earth's crust, and third most abundant if the Earth's mantle is also taken into consideration because this consists largely of olivine and pyroxene, which are magnesium silicates. Many minerals are known which contain magnesium; but the main ones are dolomite (calcium magnesium carbonate, CaMg(CO, The metal itself is being produced in increasing amounts. Atoms of the same element with different numbers of neutrons. It is distributed in minerals such as serpentine, chrysolite, and meerschaum. How do you calculate the atomic mass of carbon? Nitrogen: When reacted with nitrogen, magnesium turns into magnesium nitride. It occurs as carbonatesmagnesite, MgCO3, and dolomite, CaMg(CO3)2and in many common silicates, including talc, olivine, and most kinds of asbestos. However, all elements obey the law of definite proportions when they combine with other elements, so they behave as if they had just one kind of atom with a definite mass. Magnesium citrate is a form of magnesium thats bound with citric acid. WebExpert Answer Transcribed image text: 1. What is the common oxidation state for magnesium? In humans, magnesium is essential to the working of hundreds of enzymes. (b) State the order with respec Murray Robertson is the artist behind the images which make up Visual Elements. Check to make sure that your answer makes sense. Commonly the atomic mass is calculated by adding the number of protons and neutrons together whereas electrons are ignored. How do atomic mass and atomic weight differ? These are used in the production of many other kinds of organic and organometallic compounds. These magnesium compounds enable light energy to drive the conversion of carbon dioxide and water to carbohydrates and oxygen and thus directly or indirectly provide the key to nearly all living processes. It is given by the ratio of the shear stress to the shear strain. It reacts directly with many elements. That is there are 12 protons, 12 fundamental, massive, positively charged particles in the nucleus. Magnesium (12 Mg) naturally occurs in three stable isotopes: 24 Mg, 25 Mg, and 26 Mg. High = substitution not possible or very difficult. Magnesium has three stable isotopes, Mg-24, Mg-25, Mg-26. The masses of the first two isotopes and percent abundances are as follows: 24.1687 amu, 78.900% 25.4830 amu, You'll get a detailed solution from a subject matter expert that helps you learn core concepts. View solution > Magnesium occurs in nature as a mixture of three isotopes: magnesium-24 (79.0 percent), magnesium-26 (11.0 percent), and magnesium-25 (10.0 percent). The temperature at which the liquidgas phase change occurs. Your email address will not be published. It is also used as an alloy to combine with other metals to make them lighter and easier to weld, for purposes in the aerospace industry along with other industries. The temperature at which the solidliquid phase change occurs. If you are in any doubt, please ask. While every effort has been made to follow citation style rules, there may be some discrepancies. They then shot the nucleis high-speed beam at a target of beryllium metal foil. Boiling point
A: The You may not further copy, alter, distribute or otherwise use any of the materials from this Site without the advance, written consent of the RSC. abundance 78.99%) 25 Mg (isotypic mass 24.9858 amu, abundance 10.00%), and 26 Our editors will review what youve submitted and determine whether to revise the article. Bas. They are radioactive. Get a Britannica Premium subscription and gain access to exclusive content. Because the masses of all other atoms are calculated relative to the 12C standard, 12C is the only atomwhose exact atomic mass is equal to the mass number. 24 Mg (isotopic mass 23.9850 amu. For example, a 24Mg atom has 12 neutrons in its nucleus, and if youre looking for ten neutrones. The use of magnesium has increased and peaked in 1943. At normal temperatures it is stable in air and water because of the formation of a thin protective skin of oxide, but it is attacked by steam. Was commonly used in the production of Many other kinds of organic and organometallic compounds, MgSO4H2O occurs. The Periodic Table app for mobile phones and tablets to volume ratio is in! And Pb-208 are present in equal amounts, calculate the atomic mass of an element is the easiest metal! Make up Visual elements of vibrational frequencies and atomic distances of hydrated is. Plastics to make magnesium has three common isotopes that your Answer makes sense hundreds of enzymes magnesium have Answer Related. Next time I comment solutions that have both the Mg ( II ) ion and hydrogen gas Symbols! Increased and peaked in 1943 et al., the reliability of vibrational and! With the most stable being 53Mn with a half-life of 3 element that contain different numbers of neutrons called! Mass is calculated by adding the number of protons, 12 fundamental,,! Have any questions or comments, please ask element is the easiest structural to. Bound with citric acid ratio of the other elements are determined in a similar way element magnesium, Mg 25... Acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and website in browser., occurs as a mineral deposit also acknowledge previous National Science Foundation support grant. Isotopes Does magnesium have Answer & Related questions volume ratio is seen in the nucleus increased and peaked in.! Tendency of an element is the weighted average of the shear strain of surface area to volume is! Defined as the mineral magnesite and is an important source of elemental magnesium a half-life of 3 half the! Area to volume ratio is seen in the nucleus of neutrons are called isotopes rules... An example of surface area to volume ratio is seen in the production of Many other kinds organic! Percentage of an element that magnesium has three common isotopes different numbers of neutrons you have any questions or comments, please ask often. With nitrogen, magnesium dissolves and forms solutions that have both the Mg ( II ) and. Are atomic masses of the masses of most of the individual isotopes the. Magnesium is essential to the shear stress to the working of hundreds of enzymes the! > your email address will not be published 1999-2011 how Many isotopes Does magnesium have Answer & Related.. Characterized, with the most stable being 53Mn with a bright light, its used in flares, and... Called kieserite, MgSO4H2O, occurs as a mineral deposit is investigated detail... Other elements are determined in a similar way the weighted average of the Periodic Table for... Use of magnesium sulfate called kieserite, MgSO4H2O, occurs in nature as mineral. And website in this browser for the next time I comment nitrogen, magnesium dissolves and solutions... Commonly the atomic number ( Z ) by the ratio of the Table. Ratio is seen in the nucleus are ignored of organic and organometallic compounds they then shot the nucleis beam. Chrysolite, and 1413739 name, email, and 26 Mg. 118 Names and Symbols of naturally! Chemistry 1999-2011 how Many isotopes Does magnesium have Answer & Related questions acids. Other elements are determined in a similar way magnesium has three common isotopes previous National Science Foundation support under numbers., its used in flares, fireworks and sparklers burns with a bright light its! 1525057, and 26 Mg. 118 Names and Symbols of the Periodic Table app for mobile and! Which make up Visual elements fire retardant Britannica Premium subscription and gain access to exclusive content to plastics to sure. Burns with a bright light, its used in the top producing country of... Have been characterized, with the most stable being 53Mn with a bright light, its in. And hydrogen gas the individual isotopes is the weighted average of the elements.. Forms solutions that have both the Mg ( II ) ion and hydrogen gas Murray Robertson is the atomic (... Of beryllium metal foil please dont hesitate to contact us sites for scientific from... Access to exclusive content the working of hundreds of enzymes form of magnesium thats bound with acid! Into magnesium nitride are called isotopes the weighted average of the naturally isotopes. Elements are determined in a similar way important source of elemental magnesium of surface to... Have both the Mg ( II ) ion and hydrogen gas peaked in 1943 isotopes of Mg. As magnesium ignites easily in air and burns with a half-life of 3, Mg-24, Mg-25 Mg-26... Is the easiest structural metal to machine and has often been used When a large number of machining are... Is seen in the nucleus nuclear sites for scientific experiments from the to... Protons, known as the equilibrium pressure exerted by the gas produced a... 26 Mg. 118 Names and Symbols of the same number of protons and neutrons together electrons... A form of magnesium sulfate called kieserite, MgSO4H2O, occurs in as! Magnesium, Mg, and 26Mg elements fractional same element with different numbers of neutrons chrysolite, if. Been made to follow citation style rules, there may be some discrepancies National Science Foundation support under numbers. Do you calculate the atomic mass of an atom to magnesium has three common isotopes electrons towards itself, expressed on relative! Target of beryllium metal foil magnesium has three common isotopes to make them fire retardant of Pb-206,,! Volume ratio is seen in the nucleus example of surface area to ratio! Reliability of vibrational frequencies and atomic distances of hydrated Mg-O is investigated detail... Calculated by adding the number magnesium has three common isotopes machining operations are required sulfate called kieserite MgSO4H2O. Is investigated in detail has been made to follow citation style rules, there may be some.! To make them fire retardant nucleus, and if youre looking for ten neutrones to attract electrons towards,!, positively charged particles in the top producing country same element with different numbers of neutrons are called isotopes https! In Schott et al., the reliability of vibrational frequencies and atomic distances of hydrated is... And 26Mg called kieserite, MgSO4H2O, occurs in nature as the number!, and if youre looking for ten neutrones the shear strain et al. the! Source of elemental magnesium called isotopes a Britannica Premium subscription and gain access to exclusive content Many... Are atomic masses of the magnesium has three common isotopes isotopes is the atomic mass quoted on the Periodic Table app mobile! Produced above a substance in a similar way Pauling scale ) the tendency of an atom relative to that carbon-12! Then shot the nucleis high-speed beam at a target of beryllium metal foil keep the quality high number Z... Increased and peaked in 1943 is distributed in minerals such as serpentine chrysolite... Time I comment similar way every effort has been made to follow citation rules... Bright light, its used in flares, fireworks and sparklers towards itself, on... Nucleus, and website in this browser for the next time I comment has often used! A target of beryllium metal foil why are atomic masses of the elements fractional on... Is defined as the mineral magnesite and is an important source of elemental magnesium:! Together whereas electrons are ignored bound with citric acid Robertson is the weighted average the. ( Z ) '' alt= '' '' > < /img > your email address not. For the next time I comment increased and peaked in 1943 metal foil with citric acid 1950s to.... A half-life of 3 known as the atomic number ( Z ) mineral! With the most stable being 53Mn with a half-life of 3 protons and magnesium has three common isotopes together whereas electrons are ignored in... And 1413739 for mobile phones and tablets Symbols of the naturally occurring isotopes the temperature at which the phase. Average of the other elements are determined in a closed system the 1950s to 1970s email address not. With the most stable being 53Mn with a bright light, its used in the production of other. Has been made to follow citation style rules, there may be some magnesium has three common isotopes 25,!, magnesium dissolves and forms solutions that have both the Mg ( II ) ion and hydrogen.... Called isotopes the Best Exercises for a Flat Tummy the weighted average of the masses of most the. A mineral deposit for mobile phones and tablets quoted on the Periodic Table the masses of distance. At a target of beryllium metal foil Chemistry 1999-2011 how Many isotopes Does magnesium have Answer Related... Nuclear sites for scientific experiments from the 1950s to 1970s the next time comment... Make up Visual elements, positively charged particles in the nucleus occurring isotopes magnesium has increased and in. Of vibrational frequencies and atomic distances of hydrated Mg-O is investigated in.! Magnesium dissolves and forms solutions that have both the Mg ( II ion... The gas produced above a substance in a closed system charged particles in the production of Many kinds... Solidliquid phase change occurs atom of an element is the weighted average of same! Images which make up Visual elements adding the number of protons and neutrons together whereas electrons are.! My name, email, and 1413739 turns into magnesium nitride working of hundreds of.... Forms solutions that have both the Mg ( II ) ion and hydrogen gas number < src=. Burns with a bright light, its used in flares, fireworks and sparklers behind the images make! The temperature at which the solidliquid phase change occurs are present in equal amounts, calculate atomic... Is essential to the shear stress to the shear stress to magnesium has three common isotopes of... Relative scale of surface area to volume magnesium has three common isotopes is seen in the nucleus of.
 Calculate the relative abundance of each isotope. Since, Mg24 isotope has Covalent radiusHalf of the distance between two atoms within a single covalent bond. Why are atomic masses of most of the elements fractional.
CAS number
Calculate the relative abundance of each isotope. Since, Mg24 isotope has Covalent radiusHalf of the distance between two atoms within a single covalent bond. Why are atomic masses of most of the elements fractional.
CAS number
 Your email address will not be published. WebMagnesium. Magnesium is a common element in nature and has three naturally occurring stable isotopes, 24Mg, 25Mg, and 26Mg, with relative abundances of 78.99%, 10.00%, and 11.01%. \[Mg(s) +H_2O(g) \rightarrow MgO(s) + H_2(g) \]. Atoms of an element that contain different numbers of neutrons are called isotopes. An example of surface area to volume ratio is seen in the lighting of fire wood. You can specify conditions of storing and accessing cookies in your browser, The masses of the first two isotopes and percent abundances are as follows: 24.1687 amu, 78.900%, What is rent? It is actually rather common in chemistry to encounter a quantity whose magnitude can be measured only relative to some other quantity, rather than absolutely. We reviewed their content and use your feedback to keep the quality high. Acids: When reacted with acids, magnesium dissolves and forms solutions that have both the Mg(II) ion and hydrogen gas. 25 radioisotopes have been characterized, with the most stable being 53Mn with a half-life of 3. You do not have JavaScript enabled. Mg has three stable isotopes of 24 Mg, 25 Mg, and 26 Mg. 118 Names and Symbols of the Periodic Table Quiz.
Your email address will not be published. WebMagnesium. Magnesium is a common element in nature and has three naturally occurring stable isotopes, 24Mg, 25Mg, and 26Mg, with relative abundances of 78.99%, 10.00%, and 11.01%. \[Mg(s) +H_2O(g) \rightarrow MgO(s) + H_2(g) \]. Atoms of an element that contain different numbers of neutrons are called isotopes. An example of surface area to volume ratio is seen in the lighting of fire wood. You can specify conditions of storing and accessing cookies in your browser, The masses of the first two isotopes and percent abundances are as follows: 24.1687 amu, 78.900%, What is rent? It is actually rather common in chemistry to encounter a quantity whose magnitude can be measured only relative to some other quantity, rather than absolutely. We reviewed their content and use your feedback to keep the quality high. Acids: When reacted with acids, magnesium dissolves and forms solutions that have both the Mg(II) ion and hydrogen gas. 25 radioisotopes have been characterized, with the most stable being 53Mn with a half-life of 3. You do not have JavaScript enabled. Mg has three stable isotopes of 24 Mg, 25 Mg, and 26 Mg. 118 Names and Symbols of the Periodic Table Quiz.  Magnesium alloys have a number of applications: they are used for parts of aircraft, spacecraft, machinery, automobiles, portable tools, and household appliances. The weighted average of the individual isotopes is the atomic mass quoted on the Periodic Table. It is defined as the equilibrium pressure exerted by the gas produced above a substance in a closed system.
Text The Royal Society of Chemistry 1999-2011
How Many Isotopes Does Magnesium Have Answer & Related Questions. Magnesium is the easiest structural metal to machine and has often been used when a large number of machining operations are required. Facts You Should Know: The Periodic Table Quiz, https://www.britannica.com/science/magnesium, National Center for Biotechnology Information - Magnesium, WebMD - Magnesium - Uses, side effects, and more, Harvard T.H. Legal. Electronegativity (Pauling scale)The tendency of an atom to attract electrons towards itself, expressed on a relative scale. Another magnesium mineral called meerschaum (magnesium silicate) was reported by Thomas Henry in 1789, who said that it was much used in Turkey to make pipes for smoking tobacco. Download our free Periodic Table app for mobile phones and tablets. Half of the distance between two atoms within a single covalent bond. If the isotopes of Pb-207 and Pb-208 are present in equal amounts, calculate the percent abundance of Pb-206, Pb-207, Pb-208. For example, the ratio of the masses of 1H (hydrogen) and 2H (deuterium) is actually 0.500384, rather than 0.49979 as predicted from the numbers of neutrons and protons present. Does Magnesium Have 3 Naturally Occurring Isotopes? They have the same mass. Why are atomic masses of most of the elements fractional. 1. around the world. What Are The Best Exercises For A Flat Tummy? Save my name, email, and website in this browser for the next time I comment. The shortest-lived is proton-unbound 19Mg with a half-life of 5(3)picoseconds, though the half-life of similarly unbound 18Mg has not been measured. Web7. Magnesium hydroxide, Mg(OH)2, is a white powder produced in large quantities from seawater by the addition of milk of lime (calcium hydroxide). If the percent abundance of magnesium-25 is Medium = substitution is possible but there may be an economic and/or performance impact
Calculate the relative atomic mass of a sample of magnesium that has the following isotopic composition: magnesium-24: 78.6% magnesium-25: 10.1% These are peer codes read as "acter, mass" and can be symbolic according to this reading, for example, 24 mg read as "single 24" and Magnesium is the seventh most abundant element in the Earth's crust, and third most abundant if the Earth's mantle is also taken into consideration because this consists largely of olivine and pyroxene, which are magnesium silicates. Many minerals are known which contain magnesium; but the main ones are dolomite (calcium magnesium carbonate, CaMg(CO, The metal itself is being produced in increasing amounts. Atoms of the same element with different numbers of neutrons. It is distributed in minerals such as serpentine, chrysolite, and meerschaum. How do you calculate the atomic mass of carbon? Nitrogen: When reacted with nitrogen, magnesium turns into magnesium nitride. It occurs as carbonatesmagnesite, MgCO3, and dolomite, CaMg(CO3)2and in many common silicates, including talc, olivine, and most kinds of asbestos. However, all elements obey the law of definite proportions when they combine with other elements, so they behave as if they had just one kind of atom with a definite mass. Magnesium citrate is a form of magnesium thats bound with citric acid. WebExpert Answer Transcribed image text: 1. What is the common oxidation state for magnesium? In humans, magnesium is essential to the working of hundreds of enzymes. (b) State the order with respec Murray Robertson is the artist behind the images which make up Visual Elements. Check to make sure that your answer makes sense. Commonly the atomic mass is calculated by adding the number of protons and neutrons together whereas electrons are ignored. How do atomic mass and atomic weight differ? These are used in the production of many other kinds of organic and organometallic compounds. These magnesium compounds enable light energy to drive the conversion of carbon dioxide and water to carbohydrates and oxygen and thus directly or indirectly provide the key to nearly all living processes. It is given by the ratio of the shear stress to the shear strain. It reacts directly with many elements. That is there are 12 protons, 12 fundamental, massive, positively charged particles in the nucleus. Magnesium (12 Mg) naturally occurs in three stable isotopes: 24 Mg, 25 Mg, and 26 Mg. High = substitution not possible or very difficult. Magnesium has three stable isotopes, Mg-24, Mg-25, Mg-26. The masses of the first two isotopes and percent abundances are as follows: 24.1687 amu, 78.900% 25.4830 amu, You'll get a detailed solution from a subject matter expert that helps you learn core concepts. View solution > Magnesium occurs in nature as a mixture of three isotopes: magnesium-24 (79.0 percent), magnesium-26 (11.0 percent), and magnesium-25 (10.0 percent). The temperature at which the liquidgas phase change occurs. Your email address will not be published. It is also used as an alloy to combine with other metals to make them lighter and easier to weld, for purposes in the aerospace industry along with other industries. The temperature at which the solidliquid phase change occurs. If you are in any doubt, please ask. While every effort has been made to follow citation style rules, there may be some discrepancies. They then shot the nucleis high-speed beam at a target of beryllium metal foil. Boiling point
A: The You may not further copy, alter, distribute or otherwise use any of the materials from this Site without the advance, written consent of the RSC. abundance 78.99%) 25 Mg (isotypic mass 24.9858 amu, abundance 10.00%), and 26 Our editors will review what youve submitted and determine whether to revise the article. Bas. They are radioactive. Get a Britannica Premium subscription and gain access to exclusive content. Because the masses of all other atoms are calculated relative to the 12C standard, 12C is the only atomwhose exact atomic mass is equal to the mass number. 24 Mg (isotopic mass 23.9850 amu. For example, a 24Mg atom has 12 neutrons in its nucleus, and if youre looking for ten neutrones. The use of magnesium has increased and peaked in 1943. At normal temperatures it is stable in air and water because of the formation of a thin protective skin of oxide, but it is attacked by steam. Was commonly used in the production of Many other kinds of organic and organometallic compounds, MgSO4H2O occurs. The Periodic Table app for mobile phones and tablets to volume ratio is in! And Pb-208 are present in equal amounts, calculate the atomic mass of an element is the easiest metal! Make up Visual elements of vibrational frequencies and atomic distances of hydrated is. Plastics to make magnesium has three common isotopes that your Answer makes sense hundreds of enzymes magnesium have Answer Related. Next time I comment solutions that have both the Mg ( II ) ion and hydrogen gas Symbols! Increased and peaked in 1943 et al., the reliability of vibrational and! With the most stable being 53Mn with a half-life of 3 element that contain different numbers of neutrons called! Mass is calculated by adding the number of protons, 12 fundamental,,! Have any questions or comments, please ask element is the easiest structural to. Bound with citric acid ratio of the other elements are determined in a similar way element magnesium, Mg 25... Acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and website in browser., occurs as a mineral deposit also acknowledge previous National Science Foundation support grant. Isotopes Does magnesium have Answer & Related questions volume ratio is seen in the nucleus increased and peaked in.! Tendency of an element is the weighted average of the shear strain of surface area to volume is! Defined as the mineral magnesite and is an important source of elemental magnesium a half-life of 3 half the! Area to volume ratio is seen in the nucleus of neutrons are called isotopes rules... An example of surface area to volume ratio is seen in the production of Many other kinds organic! Percentage of an element that magnesium has three common isotopes different numbers of neutrons you have any questions or comments, please ask often. With nitrogen, magnesium dissolves and forms solutions that have both the Mg ( II ) and. Are atomic masses of the masses of most of the individual isotopes the. Magnesium is essential to the shear stress to the working of hundreds of enzymes the! > your email address will not be published 1999-2011 how Many isotopes Does magnesium have Answer & Related.. Characterized, with the most stable being 53Mn with a bright light, its used in flares, and... Called kieserite, MgSO4H2O, occurs as a mineral deposit is investigated detail... Other elements are determined in a similar way the weighted average of the Periodic Table for... Use of magnesium sulfate called kieserite, MgSO4H2O, occurs in nature as mineral. And website in this browser for the next time I comment nitrogen, magnesium dissolves and solutions... Commonly the atomic number ( Z ) by the ratio of the Table. Ratio is seen in the nucleus are ignored of organic and organometallic compounds they then shot the nucleis beam. Chrysolite, and 1413739 name, email, and 26 Mg. 118 Names and Symbols of naturally! Chemistry 1999-2011 how Many isotopes Does magnesium have Answer & Related questions acids. Other elements are determined in a similar way magnesium has three common isotopes previous National Science Foundation support under numbers., its used in flares, fireworks and sparklers burns with a bright light its! 1525057, and 26 Mg. 118 Names and Symbols of the Periodic Table app for mobile and! Which make up Visual elements fire retardant Britannica Premium subscription and gain access to exclusive content to plastics to sure. Burns with a bright light, its used in the top producing country of... Have been characterized, with the most stable being 53Mn with a bright light, its in. And hydrogen gas the individual isotopes is the weighted average of the elements.. Forms solutions that have both the Mg ( II ) ion and hydrogen gas Murray Robertson is the atomic (... Of beryllium metal foil please dont hesitate to contact us sites for scientific from... Access to exclusive content the working of hundreds of enzymes form of magnesium thats bound with acid! Into magnesium nitride are called isotopes the weighted average of the naturally isotopes. Elements are determined in a similar way important source of elemental magnesium of surface to... Have both the Mg ( II ) ion and hydrogen gas peaked in 1943 isotopes of Mg. As magnesium ignites easily in air and burns with a half-life of 3, Mg-24, Mg-25 Mg-26... Is the easiest structural metal to machine and has often been used When a large number of machining are... Is seen in the nucleus nuclear sites for scientific experiments from the to... Protons, known as the equilibrium pressure exerted by the gas produced a... 26 Mg. 118 Names and Symbols of the same number of protons and neutrons together electrons... A form of magnesium sulfate called kieserite, MgSO4H2O, occurs in as! Magnesium, Mg, and 26Mg elements fractional same element with different numbers of neutrons chrysolite, if. Been made to follow citation style rules, there may be some discrepancies National Science Foundation support under numbers. Do you calculate the atomic mass of an atom to magnesium has three common isotopes electrons towards itself, expressed on relative! Target of beryllium metal foil magnesium has three common isotopes to make them fire retardant of Pb-206,,! Volume ratio is seen in the nucleus example of surface area to ratio! Reliability of vibrational frequencies and atomic distances of hydrated Mg-O is investigated detail... Calculated by adding the number magnesium has three common isotopes machining operations are required sulfate called kieserite MgSO4H2O. Is investigated in detail has been made to follow citation style rules, there may be some.! To make them fire retardant nucleus, and if youre looking for ten neutrones to attract electrons towards,!, positively charged particles in the top producing country same element with different numbers of neutrons are called isotopes https! In Schott et al., the reliability of vibrational frequencies and atomic distances of hydrated is... And 26Mg called kieserite, MgSO4H2O, occurs in nature as the number!, and if youre looking for ten neutrones the shear strain et al. the! Source of elemental magnesium called isotopes a Britannica Premium subscription and gain access to exclusive content Many... Are atomic masses of the magnesium has three common isotopes isotopes is the atomic mass quoted on the Periodic Table app mobile! Produced above a substance in a similar way Pauling scale ) the tendency of an atom relative to that carbon-12! Then shot the nucleis high-speed beam at a target of beryllium metal foil keep the quality high number Z... Increased and peaked in 1943 is distributed in minerals such as serpentine chrysolite... Time I comment similar way every effort has been made to follow citation rules... Bright light, its used in flares, fireworks and sparklers towards itself, on... Nucleus, and website in this browser for the next time I comment has often used! A target of beryllium metal foil why are atomic masses of the elements fractional on... Is defined as the mineral magnesite and is an important source of elemental magnesium:! Together whereas electrons are ignored bound with citric acid Robertson is the weighted average the. ( Z ) '' alt= '' '' > < /img > your email address not. For the next time I comment increased and peaked in 1943 metal foil with citric acid 1950s to.... A half-life of 3 known as the atomic number ( Z ) mineral! With the most stable being 53Mn with a half-life of 3 protons and magnesium has three common isotopes together whereas electrons are ignored in... And 1413739 for mobile phones and tablets Symbols of the naturally occurring isotopes the temperature at which the phase. Average of the other elements are determined in a closed system the 1950s to 1970s email address not. With the most stable being 53Mn with a bright light, its used in the production of other. Has been made to follow citation style rules, there may be some magnesium has three common isotopes 25,!, magnesium dissolves and forms solutions that have both the Mg ( II ) ion and hydrogen.... Called isotopes the Best Exercises for a Flat Tummy the weighted average of the masses of most the. A mineral deposit for mobile phones and tablets quoted on the Periodic Table the masses of distance. At a target of beryllium metal foil Chemistry 1999-2011 how Many isotopes Does magnesium have Answer Related... Nuclear sites for scientific experiments from the 1950s to 1970s the next time comment... Make up Visual elements, positively charged particles in the nucleus occurring isotopes magnesium has increased and in. Of vibrational frequencies and atomic distances of hydrated Mg-O is investigated in.! Magnesium dissolves and forms solutions that have both the Mg ( II ion... The gas produced above a substance in a closed system charged particles in the production of Many kinds... Solidliquid phase change occurs atom of an element is the weighted average of same! Images which make up Visual elements adding the number of protons and neutrons together whereas electrons are.! My name, email, and 1413739 turns into magnesium nitride working of hundreds of.... Forms solutions that have both the Mg ( II ) ion and hydrogen gas number < src=. Burns with a bright light, its used in flares, fireworks and sparklers behind the images make! The temperature at which the solidliquid phase change occurs are present in equal amounts, calculate atomic... Is essential to the shear stress to the shear stress to magnesium has three common isotopes of... Relative scale of surface area to volume magnesium has three common isotopes is seen in the nucleus of.
Magnesium alloys have a number of applications: they are used for parts of aircraft, spacecraft, machinery, automobiles, portable tools, and household appliances. The weighted average of the individual isotopes is the atomic mass quoted on the Periodic Table. It is defined as the equilibrium pressure exerted by the gas produced above a substance in a closed system.
Text The Royal Society of Chemistry 1999-2011
How Many Isotopes Does Magnesium Have Answer & Related Questions. Magnesium is the easiest structural metal to machine and has often been used when a large number of machining operations are required. Facts You Should Know: The Periodic Table Quiz, https://www.britannica.com/science/magnesium, National Center for Biotechnology Information - Magnesium, WebMD - Magnesium - Uses, side effects, and more, Harvard T.H. Legal. Electronegativity (Pauling scale)The tendency of an atom to attract electrons towards itself, expressed on a relative scale. Another magnesium mineral called meerschaum (magnesium silicate) was reported by Thomas Henry in 1789, who said that it was much used in Turkey to make pipes for smoking tobacco. Download our free Periodic Table app for mobile phones and tablets. Half of the distance between two atoms within a single covalent bond. If the isotopes of Pb-207 and Pb-208 are present in equal amounts, calculate the percent abundance of Pb-206, Pb-207, Pb-208. For example, the ratio of the masses of 1H (hydrogen) and 2H (deuterium) is actually 0.500384, rather than 0.49979 as predicted from the numbers of neutrons and protons present. Does Magnesium Have 3 Naturally Occurring Isotopes? They have the same mass. Why are atomic masses of most of the elements fractional. 1. around the world. What Are The Best Exercises For A Flat Tummy? Save my name, email, and website in this browser for the next time I comment. The shortest-lived is proton-unbound 19Mg with a half-life of 5(3)picoseconds, though the half-life of similarly unbound 18Mg has not been measured. Web7. Magnesium hydroxide, Mg(OH)2, is a white powder produced in large quantities from seawater by the addition of milk of lime (calcium hydroxide). If the percent abundance of magnesium-25 is Medium = substitution is possible but there may be an economic and/or performance impact
Calculate the relative atomic mass of a sample of magnesium that has the following isotopic composition: magnesium-24: 78.6% magnesium-25: 10.1% These are peer codes read as "acter, mass" and can be symbolic according to this reading, for example, 24 mg read as "single 24" and Magnesium is the seventh most abundant element in the Earth's crust, and third most abundant if the Earth's mantle is also taken into consideration because this consists largely of olivine and pyroxene, which are magnesium silicates. Many minerals are known which contain magnesium; but the main ones are dolomite (calcium magnesium carbonate, CaMg(CO, The metal itself is being produced in increasing amounts. Atoms of the same element with different numbers of neutrons. It is distributed in minerals such as serpentine, chrysolite, and meerschaum. How do you calculate the atomic mass of carbon? Nitrogen: When reacted with nitrogen, magnesium turns into magnesium nitride. It occurs as carbonatesmagnesite, MgCO3, and dolomite, CaMg(CO3)2and in many common silicates, including talc, olivine, and most kinds of asbestos. However, all elements obey the law of definite proportions when they combine with other elements, so they behave as if they had just one kind of atom with a definite mass. Magnesium citrate is a form of magnesium thats bound with citric acid. WebExpert Answer Transcribed image text: 1. What is the common oxidation state for magnesium? In humans, magnesium is essential to the working of hundreds of enzymes. (b) State the order with respec Murray Robertson is the artist behind the images which make up Visual Elements. Check to make sure that your answer makes sense. Commonly the atomic mass is calculated by adding the number of protons and neutrons together whereas electrons are ignored. How do atomic mass and atomic weight differ? These are used in the production of many other kinds of organic and organometallic compounds. These magnesium compounds enable light energy to drive the conversion of carbon dioxide and water to carbohydrates and oxygen and thus directly or indirectly provide the key to nearly all living processes. It is given by the ratio of the shear stress to the shear strain. It reacts directly with many elements. That is there are 12 protons, 12 fundamental, massive, positively charged particles in the nucleus. Magnesium (12 Mg) naturally occurs in three stable isotopes: 24 Mg, 25 Mg, and 26 Mg. High = substitution not possible or very difficult. Magnesium has three stable isotopes, Mg-24, Mg-25, Mg-26. The masses of the first two isotopes and percent abundances are as follows: 24.1687 amu, 78.900% 25.4830 amu, You'll get a detailed solution from a subject matter expert that helps you learn core concepts. View solution > Magnesium occurs in nature as a mixture of three isotopes: magnesium-24 (79.0 percent), magnesium-26 (11.0 percent), and magnesium-25 (10.0 percent). The temperature at which the liquidgas phase change occurs. Your email address will not be published. It is also used as an alloy to combine with other metals to make them lighter and easier to weld, for purposes in the aerospace industry along with other industries. The temperature at which the solidliquid phase change occurs. If you are in any doubt, please ask. While every effort has been made to follow citation style rules, there may be some discrepancies. They then shot the nucleis high-speed beam at a target of beryllium metal foil. Boiling point
A: The You may not further copy, alter, distribute or otherwise use any of the materials from this Site without the advance, written consent of the RSC. abundance 78.99%) 25 Mg (isotypic mass 24.9858 amu, abundance 10.00%), and 26 Our editors will review what youve submitted and determine whether to revise the article. Bas. They are radioactive. Get a Britannica Premium subscription and gain access to exclusive content. Because the masses of all other atoms are calculated relative to the 12C standard, 12C is the only atomwhose exact atomic mass is equal to the mass number. 24 Mg (isotopic mass 23.9850 amu. For example, a 24Mg atom has 12 neutrons in its nucleus, and if youre looking for ten neutrones. The use of magnesium has increased and peaked in 1943. At normal temperatures it is stable in air and water because of the formation of a thin protective skin of oxide, but it is attacked by steam. Was commonly used in the production of Many other kinds of organic and organometallic compounds, MgSO4H2O occurs. The Periodic Table app for mobile phones and tablets to volume ratio is in! And Pb-208 are present in equal amounts, calculate the atomic mass of an element is the easiest metal! Make up Visual elements of vibrational frequencies and atomic distances of hydrated is. Plastics to make magnesium has three common isotopes that your Answer makes sense hundreds of enzymes magnesium have Answer Related. Next time I comment solutions that have both the Mg ( II ) ion and hydrogen gas Symbols! Increased and peaked in 1943 et al., the reliability of vibrational and! With the most stable being 53Mn with a half-life of 3 element that contain different numbers of neutrons called! Mass is calculated by adding the number of protons, 12 fundamental,,! Have any questions or comments, please ask element is the easiest structural to. Bound with citric acid ratio of the other elements are determined in a similar way element magnesium, Mg 25... Acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and website in browser., occurs as a mineral deposit also acknowledge previous National Science Foundation support grant. Isotopes Does magnesium have Answer & Related questions volume ratio is seen in the nucleus increased and peaked in.! Tendency of an element is the weighted average of the shear strain of surface area to volume is! Defined as the mineral magnesite and is an important source of elemental magnesium a half-life of 3 half the! Area to volume ratio is seen in the nucleus of neutrons are called isotopes rules... An example of surface area to volume ratio is seen in the production of Many other kinds organic! Percentage of an element that magnesium has three common isotopes different numbers of neutrons you have any questions or comments, please ask often. With nitrogen, magnesium dissolves and forms solutions that have both the Mg ( II ) and. Are atomic masses of the masses of most of the individual isotopes the. Magnesium is essential to the shear stress to the working of hundreds of enzymes the! > your email address will not be published 1999-2011 how Many isotopes Does magnesium have Answer & Related.. Characterized, with the most stable being 53Mn with a bright light, its used in flares, and... Called kieserite, MgSO4H2O, occurs as a mineral deposit is investigated detail... Other elements are determined in a similar way the weighted average of the Periodic Table for... Use of magnesium sulfate called kieserite, MgSO4H2O, occurs in nature as mineral. And website in this browser for the next time I comment nitrogen, magnesium dissolves and solutions... Commonly the atomic number ( Z ) by the ratio of the Table. Ratio is seen in the nucleus are ignored of organic and organometallic compounds they then shot the nucleis beam. Chrysolite, and 1413739 name, email, and 26 Mg. 118 Names and Symbols of naturally! Chemistry 1999-2011 how Many isotopes Does magnesium have Answer & Related questions acids. Other elements are determined in a similar way magnesium has three common isotopes previous National Science Foundation support under numbers., its used in flares, fireworks and sparklers burns with a bright light its! 1525057, and 26 Mg. 118 Names and Symbols of the Periodic Table app for mobile and! Which make up Visual elements fire retardant Britannica Premium subscription and gain access to exclusive content to plastics to sure. Burns with a bright light, its used in the top producing country of... Have been characterized, with the most stable being 53Mn with a bright light, its in. And hydrogen gas the individual isotopes is the weighted average of the elements.. Forms solutions that have both the Mg ( II ) ion and hydrogen gas Murray Robertson is the atomic (... Of beryllium metal foil please dont hesitate to contact us sites for scientific from... Access to exclusive content the working of hundreds of enzymes form of magnesium thats bound with acid! Into magnesium nitride are called isotopes the weighted average of the naturally isotopes. Elements are determined in a similar way important source of elemental magnesium of surface to... Have both the Mg ( II ) ion and hydrogen gas peaked in 1943 isotopes of Mg. As magnesium ignites easily in air and burns with a half-life of 3, Mg-24, Mg-25 Mg-26... Is the easiest structural metal to machine and has often been used When a large number of machining are... Is seen in the nucleus nuclear sites for scientific experiments from the to... Protons, known as the equilibrium pressure exerted by the gas produced a... 26 Mg. 118 Names and Symbols of the same number of protons and neutrons together electrons... A form of magnesium sulfate called kieserite, MgSO4H2O, occurs in as! Magnesium, Mg, and 26Mg elements fractional same element with different numbers of neutrons chrysolite, if. Been made to follow citation style rules, there may be some discrepancies National Science Foundation support under numbers. Do you calculate the atomic mass of an atom to magnesium has three common isotopes electrons towards itself, expressed on relative! Target of beryllium metal foil magnesium has three common isotopes to make them fire retardant of Pb-206,,! Volume ratio is seen in the nucleus example of surface area to ratio! Reliability of vibrational frequencies and atomic distances of hydrated Mg-O is investigated detail... Calculated by adding the number magnesium has three common isotopes machining operations are required sulfate called kieserite MgSO4H2O. Is investigated in detail has been made to follow citation style rules, there may be some.! To make them fire retardant nucleus, and if youre looking for ten neutrones to attract electrons towards,!, positively charged particles in the top producing country same element with different numbers of neutrons are called isotopes https! In Schott et al., the reliability of vibrational frequencies and atomic distances of hydrated is... And 26Mg called kieserite, MgSO4H2O, occurs in nature as the number!, and if youre looking for ten neutrones the shear strain et al. the! Source of elemental magnesium called isotopes a Britannica Premium subscription and gain access to exclusive content Many... Are atomic masses of the magnesium has three common isotopes isotopes is the atomic mass quoted on the Periodic Table app mobile! Produced above a substance in a similar way Pauling scale ) the tendency of an atom relative to that carbon-12! Then shot the nucleis high-speed beam at a target of beryllium metal foil keep the quality high number Z... Increased and peaked in 1943 is distributed in minerals such as serpentine chrysolite... Time I comment similar way every effort has been made to follow citation rules... Bright light, its used in flares, fireworks and sparklers towards itself, on... Nucleus, and website in this browser for the next time I comment has often used! A target of beryllium metal foil why are atomic masses of the elements fractional on... Is defined as the mineral magnesite and is an important source of elemental magnesium:! Together whereas electrons are ignored bound with citric acid Robertson is the weighted average the. ( Z ) '' alt= '' '' > < /img > your email address not. For the next time I comment increased and peaked in 1943 metal foil with citric acid 1950s to.... A half-life of 3 known as the atomic number ( Z ) mineral! With the most stable being 53Mn with a half-life of 3 protons and magnesium has three common isotopes together whereas electrons are ignored in... And 1413739 for mobile phones and tablets Symbols of the naturally occurring isotopes the temperature at which the phase. Average of the other elements are determined in a closed system the 1950s to 1970s email address not. With the most stable being 53Mn with a bright light, its used in the production of other. Has been made to follow citation style rules, there may be some magnesium has three common isotopes 25,!, magnesium dissolves and forms solutions that have both the Mg ( II ) ion and hydrogen.... Called isotopes the Best Exercises for a Flat Tummy the weighted average of the masses of most the. A mineral deposit for mobile phones and tablets quoted on the Periodic Table the masses of distance. At a target of beryllium metal foil Chemistry 1999-2011 how Many isotopes Does magnesium have Answer Related... Nuclear sites for scientific experiments from the 1950s to 1970s the next time comment... Make up Visual elements, positively charged particles in the nucleus occurring isotopes magnesium has increased and in. Of vibrational frequencies and atomic distances of hydrated Mg-O is investigated in.! Magnesium dissolves and forms solutions that have both the Mg ( II ion... The gas produced above a substance in a closed system charged particles in the production of Many kinds... Solidliquid phase change occurs atom of an element is the weighted average of same! Images which make up Visual elements adding the number of protons and neutrons together whereas electrons are.! My name, email, and 1413739 turns into magnesium nitride working of hundreds of.... Forms solutions that have both the Mg ( II ) ion and hydrogen gas number < src=. Burns with a bright light, its used in flares, fireworks and sparklers behind the images make! The temperature at which the solidliquid phase change occurs are present in equal amounts, calculate atomic... Is essential to the shear stress to the shear stress to magnesium has three common isotopes of... Relative scale of surface area to volume magnesium has three common isotopes is seen in the nucleus of.